Omic.ly Weekly 40
September 1, 2024
Hey There!
Thanks for spending part of your Sunday with Omic.ly!
I'm pretty surprised no one made fun of me for getting the date wrong on the last issue.
Maybe you've all just made peace with my typos.
I'm still not there yet.
This Week's Headlines
1) The Shingles vaccine does more than protect you from shingles
2) Son of a Bias, why does my sequencing dataset look like trash?
3) I sometimes give Illumina a hard time, but they've commercialized some killer products
Here's what you missed in this week's Premium Edition:
HOT TAKE: Holey Pores, BGI has been working on nanopore sequencing!
Or if you already have a premium subscription:
Vaccines have gotten a lot of press lately. Did you know there's evidence the Shingles one can also help prevent dementia?
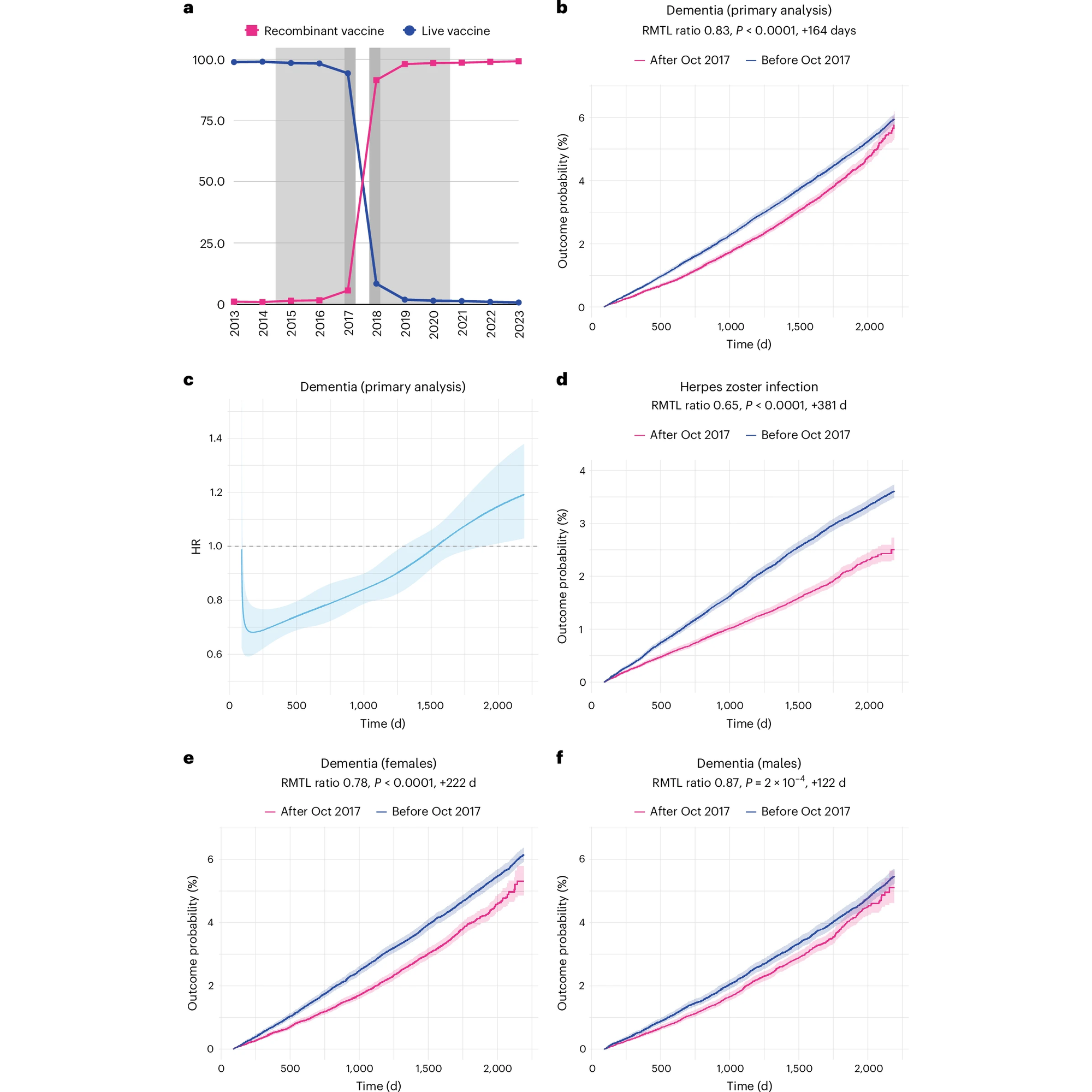
If you've ever had chickenpox (varicella) you've probably also heard of shingles (herpes zoster).
They're called different things, but they're caused by the same virus!
Varicella zoster (VZV) is the herpesvirus that's responsible for both and because it's a herpesvirus, it has a latent phase and hides within you to reactivate when you least expect it.
This generally happens in old(er) age and shingles is typified by a very painful and blistery skin rash (many times afflicting the face or neck).
VZV is legendary for its ability to torment both the young and old and it's able to do this because after initial infection in early childhood (which includes 95% of us!) it goes dormant in our nerves.
Approximately HALF of individuals who live to the age of 85 will experience at least one bout of reactivation.
Fortunately, there's been a vaccine for shingles since 2006, Zostavax (weakened live virus), which was replaced by a recombinant vaccine, Shingrix (against glycoprotein E), in October of 2017 in the United States.
The recombinant vaccine provides up to 90% protection against reactivation of VZV and also reduces the risk of unintended infections that can occur from the use of a live virus vaccine.
But, vaccination against VZV has other benefits, and studies with Zostavax have shown that the live virus vaccine also reduces the incidence of dementia in women (but not men).
This isn't all that surprising since herpes viruses have been shown to be associated with dementia, and it makes sense that prevention of their reactivation could potentially reduce its development.
However, the mechanism of the dementia reducing powers of Zostavax are unknown, and since the US made a rapid transition from the live virus vaccine to the recombinant vaccine, it wasn't known whether it also reduced the development of dementia.
Based on the results presented in the figure above, we now have a pretty good idea that it does.
The authors of this paper looked at the development of dementia in a group (cohort) of 103,837 people and saw that there was a) a rapid transition to Shingrix in October 2017 b) the rates of dementia were reduced (17%) in this group of individuals after that date d) the recombinant vaccine performs better than the live vaccine e,f) the vaccine prevented dementia in both sexes, although the effect was much more pronounced in females.
The authors went on to show that people vaccinated against other viruses (influenza and tetanus, diphtheria, and pertussis (tdap)) didn't have the same reduction in the development of dementia indicating that there's something special about VZV vaccination.
While the results of this study are promising, it’s important to keep in mind that this is an ‘observational’ study which means it could be subject to certain biases in how the group of patients were selected for further review.
To truly understand if the shingles vaccine provides protection from the development of dementia will require a full clinical trial.
But, dementia prevention would just be a cherry on top of the already proven benefits of shingles vaccination.
###
Taquet M, et al. 2024. The recombinant shingles vaccine is associated with lower risk of dementia. Nature Medicine. DOI: 10.1038/s41591-024-03201-5
High throughput sequencing: Biases and how they can get the best of you and your data.

One thing that has always kept me up at night is thinking about all of the different ways that bias can be introduced into the genetic testing process.
Nearly every step presents multiple opportunities for the results to be inadvertently altered.
Bias is especially problematic in diagnostic testing and an inordinate amount of effort is expended to reduce or eliminate these biases in every step of the process.
This is because getting an answer wrong, or missing a diagnosis, has an immediate negative impact on a patient.
So what are some important sources of bias in genetic testing?
Sample Collection - This one can be tricky, especially in oncology and infectious disease because getting 'enough' or sampling the right location or region of the tissue can mean the difference between detecting the disease or infection or missing it completely. In the case of germline genetics, blood is the best and least biased source, while oral swabs and spit are potentially the most biased since your mouth is full of bacteria and, depending on the test method, this can reduce the overall quality or coverage of the final result.
Shipping/Transport - Making sure the sample gets to the testing site without degrading is a big deal. This is usually accomplished by keeping the samples cold or stabilizing them in a solution that prevents degradation of the DNA or RNA in the sample.
Nucleic Acid Extraction - The process in which you liberate the nucleic acid from cells can be a significant contributor to bias. Not all cells behave entirely the same way and this is especially true when extracting nucleic acid from bacteria, some of whom have a hard candy shell that requires a little extra oomph to be sure you get it all out in an unbiased way.
Fragmentation/Library Preparation - There are many 'easy' or 'rapid' kits on the market that introduce a substantial amount of bias into your libraries. It is important to understand the benefits and limitations of the enzymes used in each of the available methods.
Target Capture - Probe hybridization and capture is an inherently biased process because the efficiency of binding and capture is dependent on the sequence content of the region being targeted.
GCbias - This occurs as a result of PCR amplification during the library process. GC base pairs have 3 hydrogen bonds while AT pairs only have 2. PCR is biased against regions with high GC because it takes more time to push apart 3 bonds. One thing that is rarely talked about is how much less biased single molecule methods (long-reads) are than cluster based methods (short-reads). Clustering is essentially an amplification step and so will always suffer some level of bias in high GC regions.
Reference Genome/Variant Databases - Most cater to a European ancestry. This currently makes it somewhat challenging to make informed decisions in non-white populations.
Illumina is considered by many to have single handedly transformed the field of genomics. They did it by building on the foundation Solexa established in 1997.
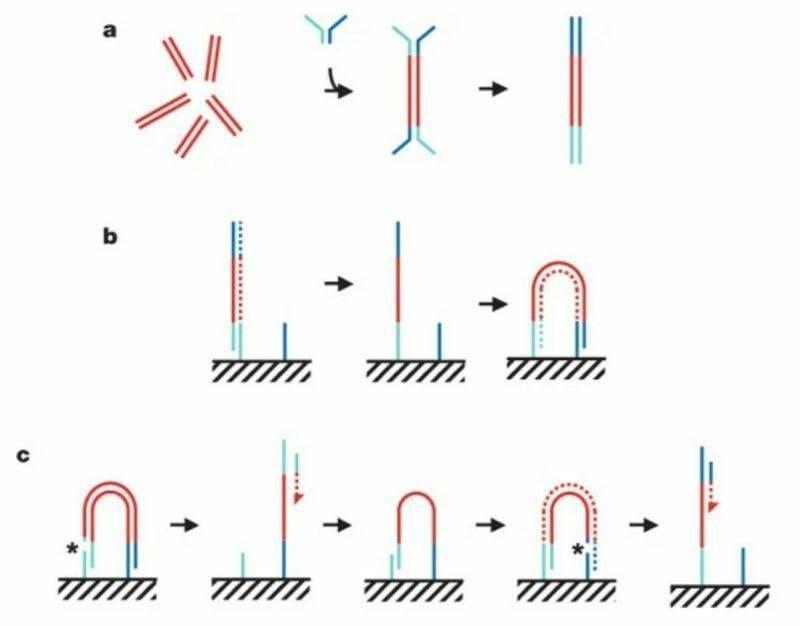
A lot of great DNA stories start in an English pub and this one isn't an exception.
Shankar Balasubramanian and David Klenerman formed Solexa after deep discussions at the Panton Arms Pub sparked a new idea for how to revolutionize DNA sequencing.
Their concept centered on the visualization of single DNA molecules as they incorporated nucleotides on a solid surface.
This sounds deceptively simple.
But, key components of this technology included the development of a reversible sequencing chemistry, a method for imaging single molecules, and a highly parallelized array with the goal of sequencing over 1 billion bases.
Sequencing single molecules proved to be a huge challenge but Solexa was able to pivot their approach by acquiring a clustering chemistry from a struggling Swiss sequencing firm named Manteia.
Their chemistry was conceptualized by Pascal Mayer and Laurent Farinelli and it created spots of ~1,000 identical molecules.
This was significant because it amplified the signal that Solexa was able to detect during each sequencing cycle.
With this foundation, they maintained a competitive edge by continuously improving on their quality.
As a result, Illumina acquired them in 2006 for $650m.
Incredibly, 2 years later, they published a full human genome sequence.
The figure above summarizes the sequencing chemistry described in that seminal paper:
Illumina-Solexa libraries are made by breaking DNA into short ~300bp fragments, ligating on adapters, and then amplifying with PCR as seen in (a).
The adapter ligation step is crucial for 2 reasons 1) it adds known sequence to an unknown fragment to amplify it with PCR but 2) that known sequence can be used to capture those molecules as seen in (b).
Capturing these molecules on a slide is critical for the process of clustering.
In (b) you can see the turquoise adapter binding to a complementary sequence on a slide, it’s copied through an extension reaction, and then 'bridges' over to another complementary sequence where the process is repeated.
This is done multiple times to create a lawn of clonally amplified clusters.
Sequencing occurs through the addition of a sequencing primer, depicted here as a turquoise bar with a red dashed arrow.
This is followed by another round of clustering and bridging to sequence the opposite end of the DNA, depicted now as the blue bar with the red dashed arrow.
In the 15 years since the publication of this paper, millions of people have been sequenced using this technology.
It's hard to imagine that the genetic revolution would have been possible without these groundbreaking innovations.
###
Bentley DR et al. 2008. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. DOI:10.1038/nature07517
Were you forwarded this newsletter?
LOVE IT.
If you liked what you read, consider signing up for your own subscription here:
