Omicly Weekly 1
December 3, 2023
Hey There!
Thanks for being a BETA Omic.ly guinea pig!
I would really appreciate it if you could send me any feedback you might have about this or any of the other BETA newsletters over the next 3 weeks.
Omicly.Contact@gmail.com
Please enjoy!
In this week's newsletter you will find:
1) My 3 predictions for all things omics in 2024
2) Why cutting edge drug therapeutics should be paired with multi-omics
3) The story of Rosalind Franklin and photo 51
Here are my 3 predictions for all things Omics in 2024:
1. The Short-Read Market Sees Some Consolidation
Given the current economic climate and, more importantly, the climate in diagnostics and the life sciences, it seems inevitable that someone in the short read market is going to go bankrupt or get acquired.
I might go as far as to say that I don't think anyone is safe here…
Ok, that's not exactly true. I think Complete Genomics will be fine because they're a subsidiary of a much larger and well funded organization. They also have a pile of patent judgment money from Illumina so I think they're probably the safest.
But I actually think everyone else is on the bubble!
Both Illumina and Element are attractive acquisitions. Illumina because they're Illumina and are currently sitting at about double what Roche offered for them back in 2012. They also have a number of big payments coming up, and if Grail is divested they become even cheaper. It could be a good time for an opportunist to swoop in!
Element is really the only other company that has actual customers so if there's someone that wants to take the fight to Illumina and Complete, supercharging Element might be able to do some damage!
That said, I still think Singular and Ultima are going to struggle. Singular because the market has kind of already told them they're not interested, and Ultima because they're coming in with a pricey CAPEX instrument and technology that is unproven.
2. Proteomics Hits Commercialization Warp Speed
I'm super bullish on the future of proteomics and Thermo’s purchase of Olink hints at a bright future. Thermo usually only buys things that they think they can commercialize quickly and that's what I anticipate seeing over the next year.
I'm also encouraged by the continuous expansion of targets that are offered from both SomaLogic and Olink. These two provide the nearest term opportunity to bring proteomics to the clinic.
On the flip side, I think everyone in the startup space is going to struggle throughout 2024. I do hope many of the early stage protein sequencing and mass spec companies survive because I don't think multiplex immunoarrays are the ideal version of proteomics!
3. Labs Will Continue to Pretend the FDA Isn’t a Problem for Them
The deadline for submitting comments to the FDA about their new rule to regulate lab tests is Dec 4.
What this means is that FDA is likely to formulate their responses to those comments and move forward with congressional review of the rule next year.
If you're holding out hope that it's blocked, I think you're going to be disappointed.
And then the clock starts ticking...
I also don't think the industry titans are going to fight very hard. They're in the best position to mop up the customers left behind by the labs that wait too long to take this seriously!
That's the benefit of running IVDs and having everything NYS or FDA approved.
The drug therapeutic landscape expands almost daily. Pairing them with multi-omics is a no brainer!

That isn't rocket science.
But what IS rocket science is developing the tests that make sure those therapies work, and continue to work, when administered to patients!
We do have some pretty good experience with this sort of thing already though.
Technologies such as pharmacogenetic testing have emerged to help predict how patients will respond to certain classes of drugs.
This is done by looking at genetic markers that indicate how quickly someone might metabolize, absorb, or eliminate a drug!
But we also have experience developing ‘companion diagnostics.’
These are diagnostic tests that are used to place patients on a specific therapy.
The first of these was used in 1998!
HercepTest was introduced to identify patients who would respond best to Herceptin, an early antibody treatment for breast cancer.
This test was necessary because Herceptin only worked in patients if their tumors overexpressed the HER2 receptor!
And because of this integration with a biomarker test, Herceptin is often referred to as the poster child for precision medicine!
But we’ve come a long way since Herceptin, and there are some really cool new precision therapeutics on the horizon:
PROteolysis TArgeting Chimeras (PROTACs) - Small molecule drugs that have one end that binds to a target protein and another that binds to E3 Ubiquitin Ligase (a protein that marks other proteins for destruction!)
Antibody Drug Conjugates (ADCs) - These are antibodies that are physically bound to drugs to make their delivery more targeted. The biggest successes here have been in targeting chemotherapy drugs to tumors!
Translation Activating RNAs (taRNAs) - RNA molecules designed to bind to a target RNA to supercharge its translation. This is done by adding a sequence called an Internal Ribosome Entry Site (IRES). These boost ribosome binding on the target RNA and increases production of the target protein.
mRNA Vaccines - We're all familiar with these, but what you might not know is that they can also be quickly programmed to create personalized cancer treatments.
Chimeric Antigen Receptor - T cells and Macrophages (CAR-T/M) - Are immune cells that have been programmed or personalized to seek out and destroy tumor cells.
These are all very exciting, but their development requires a lot of testing to tailor each treatment to an individual.
But what excites me the most in this space is getting the opportunity to move beyond the single biomarker tests of old!
Because seeing the full picture of a patient's response to a therapy through expanded proteomic and metabolomic screening could:
1) Show us how well a drug is working
2) Signal when someone will relapse
3) Mitigate side effects before they're felt
4) Indicate when to change therapies
This is the version of precision medicine that we were promised and I’m hopeful we see these applied more broadly in the clinic soon!
The most famous photo in the history of genetics wasn’t generated by Watson and Crick, but that didn’t stop them from using it to solve the structure of DNA!
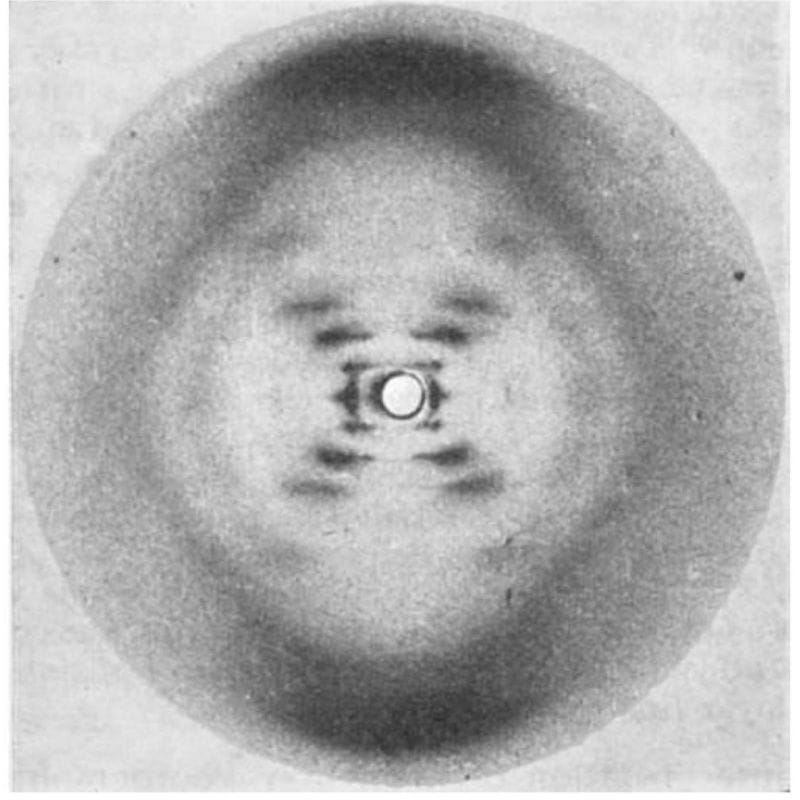
While they tend to get all of the credit for this structure, 3 papers were published back-to-back on this topic in April of 1953:
1) Watson and Crick’s has a single, hand-drawn, 3D structure of DNA.
2) Wilkins' includes a blurry diffraction pattern.
3) Franklin and Gosling's has the pristine diffraction pattern of DNA known as Photo 51. Their analysis showed that the structure is helical, it's double stranded, and the bases face inward with the phosphate backbone on the outside.
So, why are there 3 papers?
In the early 1950’s, scientists were coming around to the idea that DNA was the genetic material, but the publication of the Hershey-Chase experiment in 1952 removed all doubt.
Soon after, Linus Pauling, an American structural biologist, published a triple-helical structure of DNA (with the bases facing outward).
Unfortunately for Pauling, his structure was based on flawed data but this put the teams in the UK on notice.
Watson and Crick were at Cambridge while Wilkins, Franklin and Gosling were at King’s College London.
Wilkins did much of the early work generating x-ray diffraction patterns of DNA, but it was Franklin and her PhD student, Gosling, who perfected the art.
In May of 1952, Gosling generated photo 51 of B-DNA, but Franklin was more interested in studying A-DNA.
So, photo 51 wasn’t revisited until she decided to leave King’s.
Since Gosling was transferring to Wilkins to complete his PhD, photo 51 was shared with him and he in turn shared it with Watson.
Realizing this fixed all of their problems, he and Crick completed the model for their structure of DNA, culminating in the publication of all 3 papers in 1953.
Unfortunately, Franklin died of ovarian cancer in 1958 and Watson, Crick and Wilkins won the Nobel in 1962.
The story of photo 51 is incredible, but what’s even more incredible is that Franklin’s contribution was nearly forgotten.
This changed when Watson’s misogyny got the better of him.
In his 1968 memoir, he composed a very unkind depiction of Franklin (He apologized in a later edition).
This sparked renewed interest in her and corrected portrayals of her and her work have been published:
She was a pioneer of x-ray crystallography.
She provided the key diffraction pattern for the structure of DNA.
She resolved the structure of RNA in Tobacco Mosaic Virus.
And, prior to her death, she worked on polio virus.
Her collaborator, Aaron Klug, finished this project and was awarded the Nobel Prize, in part, for his crystallography work.
Though short a Nobel (or two), Rosalind Franklin's scientific legacy is extraordinary.
###
Franklin RE and Gosling RG. 1953. Molecular Configuration in Sodium Thymonucleate. Nature. DOI: 10.1038/171740a0
