Green Fluorescent Protein was first discovered in Jellyfish in 1960
In 1994, an obscure jellyfish protein was stuck into a worm. It has enlightened biology and medicine ever since.
In 1994, an obscure jellyfish protein was stuck into a worm. It has enlightened biology and medicine ever since.
But before we get to the genetically engineered worm, we first have to talk about the discovery of that jellyfish protein in 1960.
Aequorea aequorea, the jellyfish species in question, might seem like an odd place to start, but jellyfish are bioluminescent.
Osamu Shimomura was interested in studying the chemistry and physics of how these animals were able to produce their blue-green glow.
Up until that point, it was known that most bioluminescent creatures, like fireflies, do so with an enzymatic reaction between luciferin and luciferase.
But Shimomura discovered something different!
And in 1962 he isolated aequorin, a calcium binding protein that glows blue, along with another protein that had a bright green color under UV light.
Over the next decade, Shimomura dissected how aequorin and the green protein worked together to produce the characteristic glow of these jellyfish.
As it turns out, the blue calcium binding protein excites the green protein!
And in 1974 he published a paper describing what we now know of as green fluorescent protein (GFP).
Although Shimomura discovered the protein, he didn't see any further use for it and so focused his research elsewhere.
However, a few years later, this work was picked back up by Doug Prasher.
Unfortunately, getting a hold of enough protein to do anything useful with it was challenging because it had to be harvested from animals.
Prasher got the bright idea that he could use a new technique called cloning to make a bunch of this protein without having to collect and dissect a ton (literally) of jellyfish.
He was the first to sequence the GFP gene, but he couldn't get the cloned protein to light up, and so he gave up after publishing a paper about cloning the gene.
Soon after, Prasher was contacted by two labs who saw his paper and were interested in using GFP as a molecular tracer for imaging.
Martin Chalfie and his rotation student, Ghia Euskirchen, got the first shot and found that the original clone didn't work because it contained a couple of extra amino acids that prevented the protein from properly folding.
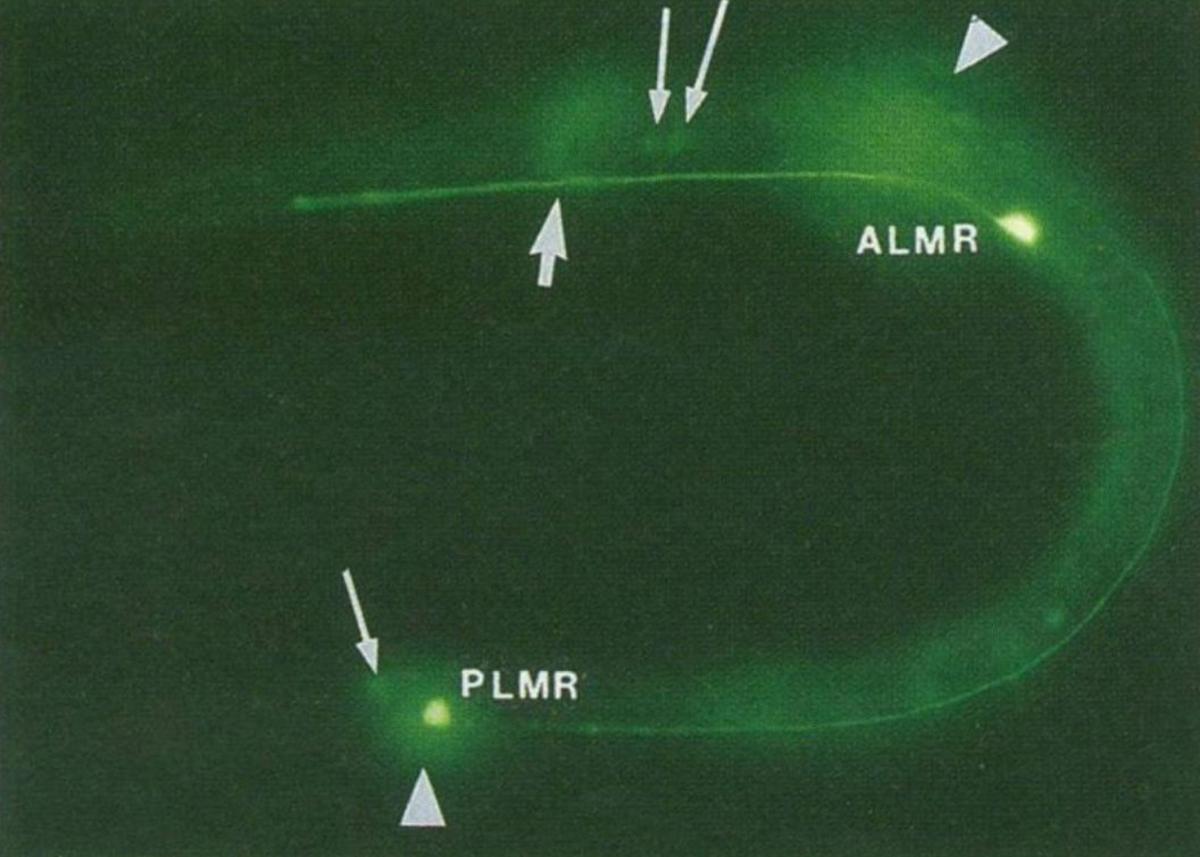
The figure above is the result of putting that now functioning sequence into C. elegans, a nematode worm.
It was expressed using an ALM/PLM neuron specific promoter (bright green dots).
But the story doesn't end there!
The second lab to get Prasher's GFP clone was Roger Tsien's.
Tsien was a biochemist and had visions for GFP beyond just green ones.
His lab created much of the fluorescent protein rainbow that we have available today.
Shimomura, Chalfie, and Tsien shared the Nobel Prize for this work in 2008.
And fluorescent proteins are now a ubiquitous molecular tool that color everything from cell cultures to aquarium fish!