The epigenome's role in aging gets some mechanistic answers
Feeling old? It might be time to blame your epigenome.
The link between DNA methylation, the epigenome and aging isn’t new.
We’ve known for some time that DNA methylation is important for controlling which genes get expressed.
DNA methylation, and epigenetics more broadly, are important for cellular differentiation and cell fate decisions during development.
Maintaining epigenetic marks is required for the proper function of tissues in multi-cellular organisms.
So, it stands to reason that disruption of those epigenetic marks as we age could have important consequences!
This is supported by evidence from studying aging in model organisms like yeast, fruit flies and naked mole rats.
These results seem to suggest that aging probably isn’t caused by the accumulation of DNA mutations over time.
Instead, changes to epigenetic factors, specifically those linked to regulating DNA methylation, have been shown to increase lifespan in many of these model systems.
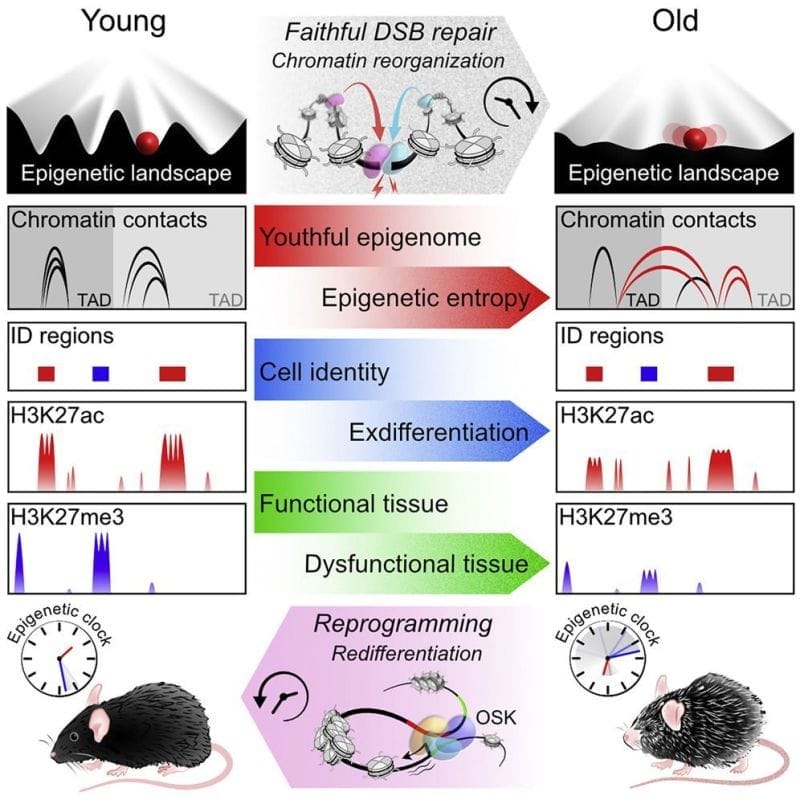
Interestingly, these same factors have also been found to help to fix the genome when errors or breaks are found.
This makes sense, because we know that maintaining the epigenome is important for cellular function, so if there’s a break that needs to be repaired, the epigenetic marks in that repaired region also need to be re-established for proper function!
It’s estimated that up to 50 DNA breaks occur in every cell every single day, and over a cell’s lifespan, that’s a lot of repairs.
The author’s of today’s paper hypothesized that there was a link between these DNA repair functions and the loss of epigenetic control as we age.
They propose the ‘‘Information Theory of Aging’’ stating that, “aging in eukaryotes is due to the loss of transcriptional networks and epigenetic information over time, driven by a conserved mechanism that evolved to co-regulate responses to cellular damage...”
To test this hypothesis they developed a model system in mice where they could induce DNA strand breaks without causing mutations in any genes.
They quantified these effects molecularly by looking at changes in RNA expression, epigenetic regulatory marks and chromatin contacts.
Overall, the results show that these mice exhibit features of old age, and they link DNA repair to accelerated epigenetic aging.
Interestingly, the researchers showed in a follow-up experiment that activating Oct4, Sox2, and Klf4 (ES cell fans will be familiar with these already) in aged mice reversed the gene expression changes.
The main takeaway here being that excessive DNA damage repair and a loss of epigenetic information can cause aging but that this loss is potentially reversible.
Now we just need to find a safe and effective way to induce those epigenetic reset genes in every cell of our body.
