Contrary to popular belief, there are 21 amino acids and the 21st was discovered in 1976
You might have been told that there are 20 amino acids. That’s a lie.
There are actually about 500 that occur in nature.
But only 22 of them are found in proteins across all organisms.
Of those, 21 appear in humans!
And I know it's killing you to know the name of the 21st amino acid.
But you're going to have to meet Thressa “Terry” Stadtman first.
Stadtman studied two anaerobic bacteria, Clostridium sticklandii and Methanococcus vannielii.
She isolated them from mud that she collected on the shore of San Francisco Bay in 1940.
They served her dutifully throughout her career.
She was fascinated by these microbes because they seemed to be like little chemical magicians!
They were able to perform chemistry at room temperature that would be nearly impossible in a traditional lab.
And they did this chemical magic using enzymes!
One of these was glycine reductase which converts the amino acid glycine to acetyl-phosphate (AcP).
AcP can recharge ADP and turn it into ATP.
If you don’t speak cellular metabolism, this means AcP can be used to literally make energy!
Stadtman was trying to figure out how this worked, but noticed that every so often her bacteria weren’t able to reduce glycine.
She discovered that this occurred whenever one of the components of her enzyme system was missing: protein A.
Thinking this was likely due to a deficiency in the food she used to grow her bacteria, she tested whether the addition of certain nutrients was able to fix the problem.
She found that dumping in Selenium, a trace element, restored protein A production!
But, that posed another question:
Why?
Stadtman decided to answer this by growing her bacteria on radioactive Selenium (75-Se) so she could see where it was going.
She discovered that it actually ended up IN protein A!
She published this finding in 1974, referring to protein A as a ‘selenoprotein.’
But this created another question:
Where was Se hiding?
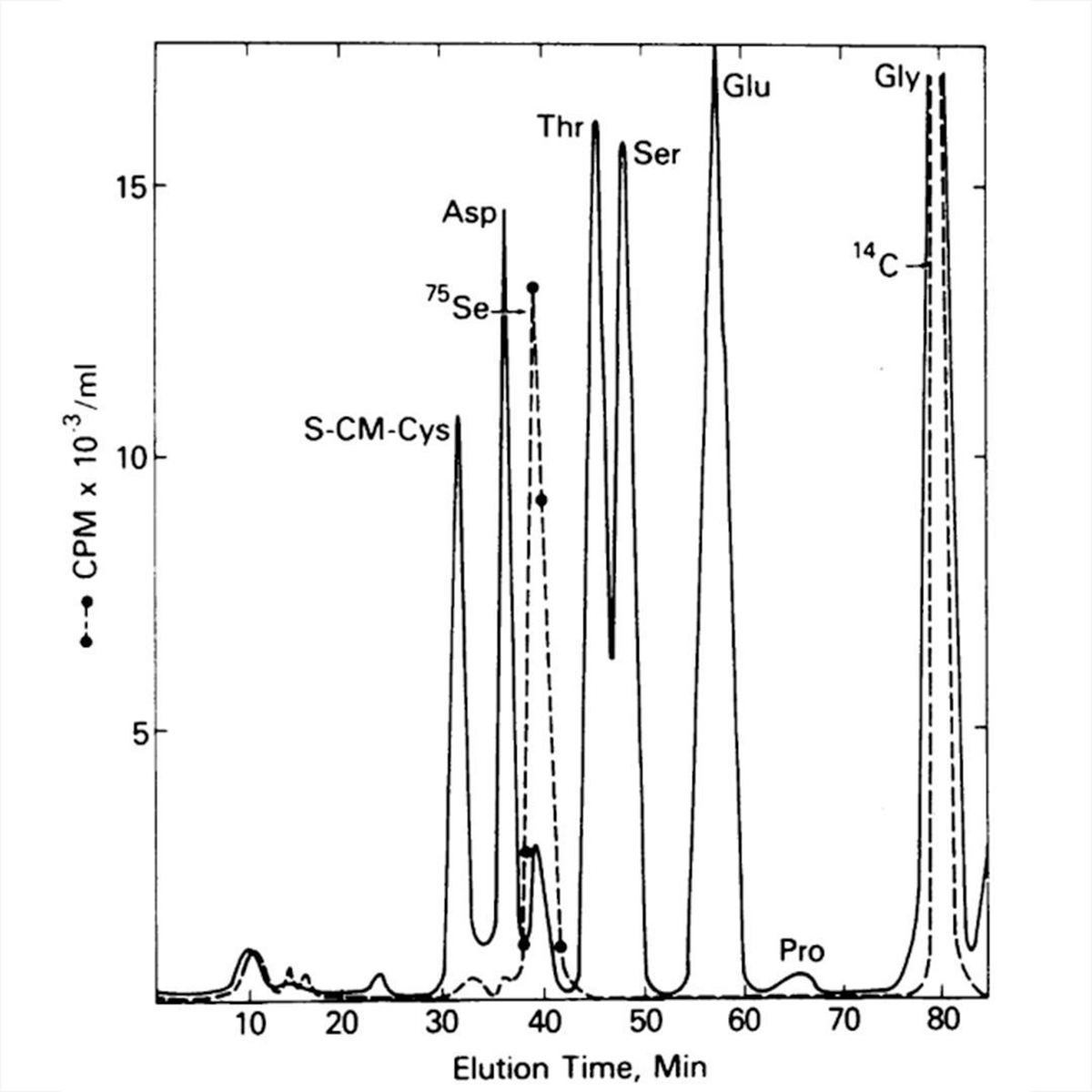
The figure above provides a pretty definitive answer.
Stadtman grew her bacteria on 75-Se again, isolated protein A, digested it down to its amino acid components, and threw that on a chemical analyzer!
In the plot, the solid line identifies the amino acid components of protein A and the dashed line tracks the radioactive signal.
Stadtman saw that the 75-Se peak coincided with a small unidentified amino acid peak after Aspartate (Asp).
The new peak was too close to Asp to be an Se derivative but she had a hunch that it was a new form of cysteine (S-CM-Cys) which appears first.
This was confirmed in a follow-up experiment!
Not only had Stadtman discovered where the Se went, she also discovered the 21st amino acid:
Selenocysteine!
It was later shown that this rare amino acid is coded for by the UGA stop codon and a special hairpin sequence at the end of a handful of RNAs.