APOE4: no longer just a risk factor for Alzheimer’s, it's a genetically distinct form of the disease!
This genetic distinction bears the gift of new tools to predict onset and track disease progression.
Alzheimer’s disease (AD) is the cause of ~ 70% of the cases of dementia in the US.
It's also the seventh leading cause of death.
The cause of AD is still largely unknown although there are genetic and environmental factors that contribute to the disease.
It is typified by the development of plaques in the brain that lead to the progressive loss of brain function.
The plaques are caused by the abnormal accumulation of the proteins amyloid beta (Aβ) and Tau.
Deficiencies in clearing these proteins are thought to result in inflammation of the brain that interferes with the connections between neurons and, ultimately, their death.
The best evidence we have for this mechanism is that the gene that leads to the creation of Aβ, Amyloid Precursor Protein (APP), is found on chromosome 21 and people with Down Syndrome (three copies of 21) generally show symptoms of AD by the age of 40.
This is further supported by Autosomal Dominant AD which is caused by mutations in APP and the proteolytic proteins PSEN1 and PSEN2.
These result in the abnormal overproduction of Aβ.
But, greater than 90% of cases of AD are sporadic (no family history or obvious genetic cause), although there are a number of genes that are risk factors for the disease.
One of those is Apolipoprotein E (APOE) which is involved in transporting lipids.
Genetic studies have identified 3 variants of APOE in humans: ε2, ε3, and ε4.
APOEε4 (or just APOE4) is associated with problems in regulating lipid metabolism and is often seen in people with hypercholesterolemia (too much cholesterol) and hyperlipidemia (too much fat in the blood).
It has also been associated with up to 80% of sporadic cases of AD and people who carry two copies of APOE4 have a 60% lifetime risk of developing disease.
While a lot of questions still remain about how APOE4 can cause AD, the author’s of today’s paper make a very compelling argument that APOE4 represents a genetically distinct form of AD showing “near-full penetrance, symptom onset predictability and a predictable sequence of biomarker and clinical changes” that lead to disease.
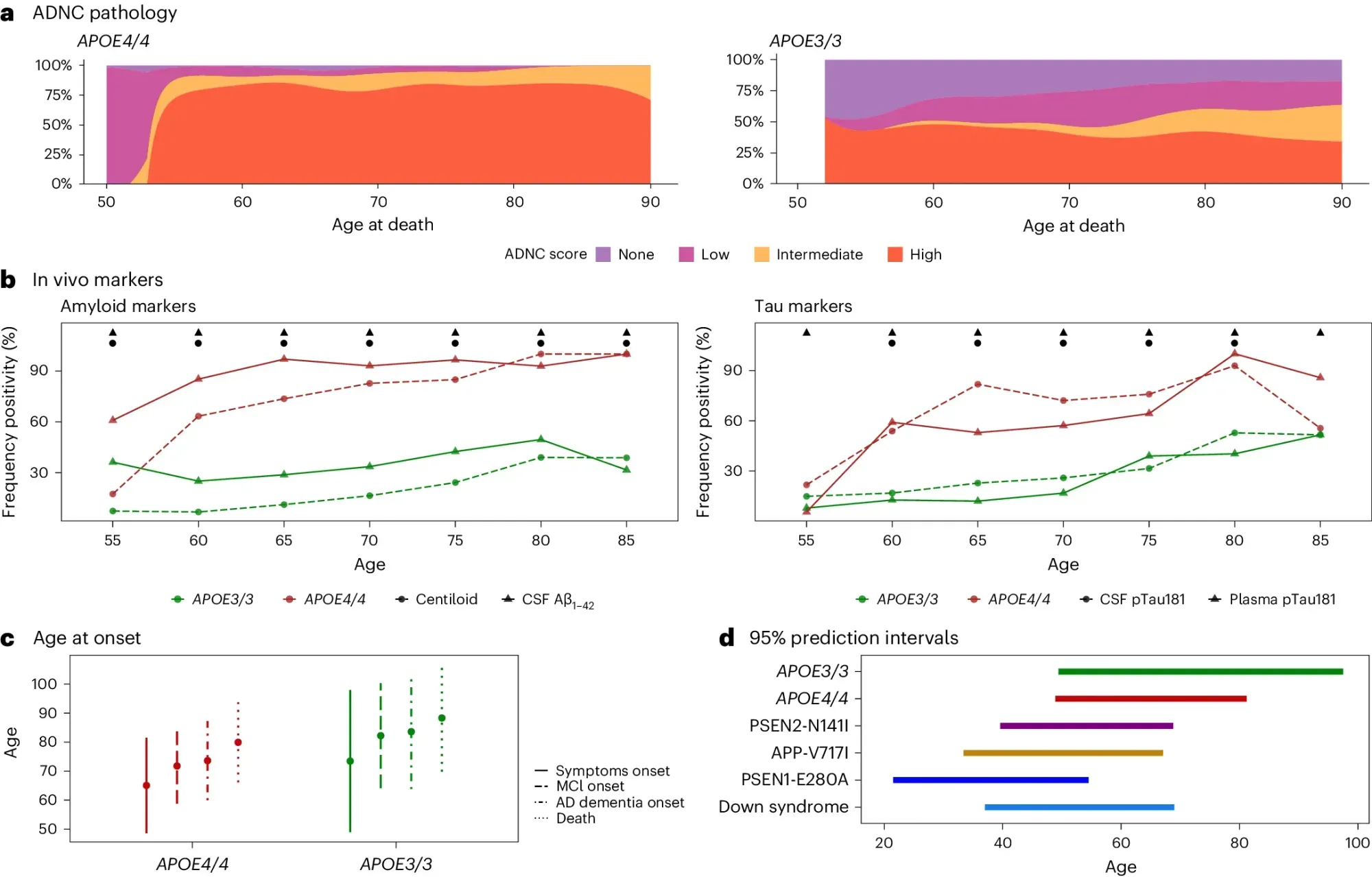
They looked at the brains of >3,000 deceased AD patients and the clinical presentation of >10,000 individuals living with AD.
What they found (see the figure above) is that APOE4/4 AD patients a) have intermediate to high levels of neuropathology, b) >90% show biological hallmarks of the disease by age 65, c and d) have earlier disease onset, comparable to the other genetically distinct forms of AD.
This genetic distinction is important because it allows us to better inform patients of their risk, and gives us new tools to predict onset and track disease progression.