AI is making new, functional, enzymes now
Artificial intelligence has been used to develop a new enzyme that can catalyze a multistep chemical reaction!
Enzymes are like little chemistry magicians.
They make complex chemical reactions happen at room temperature that would be nearly impossible to perform outside of an advanced laboratory.
And they’re able to perform these amazing chemical feats because enzymes work by grabbing onto molecules and forcing them together (or pulling them apart) at the molecular level.
All of this happens within the “active site” of an enzyme which is a cavity within the protein that interacts with the molecules that are participating in the chemical reaction.
But enzymes don’t JUST force molecules together, sometimes they become intermediaries within the process and covalently bond to these molecules to get them into the right state to perform the next step in the reaction.
That is to say, this is all really complex and it’s a wonder that enzymes have evolved to be able to perform these fantastic feats.
It also means that designing our own enzymes is incredibly tough, and until recently our best techniques for building new enzymes involved processes like “directed evolution” or just plain old trial and error.
Directed evolution is where you take an organism (usually a bacterium), expose it to nasty conditions containing the molecule you want it to deal with, and through multiple rounds of selection, find a bacterium that eventually develops useful enzymes that do the thing you want them to do.
But this is a time consuming process and rarely ends up producing enzymes that are highly efficient!
There has to be a better way, right?!
That better way for a long time seemed like it was going to involve computational biology, but even using complex supercomputers, we couldn’t really develop useful enzymes that could perform multi-step chemical reactions!
Usually what got made could perform single steps, but then they would be gummed up in the process before moving on to the next step of the reaction.
That’s super frustrating!
However, we now have AI algorithms that we can solve these computational problems and we can employ them to help us iterate through candidate proteins that might be able to help us produce enzymatic magicians of our own.
In this week’s paper, researchers attempted to use AI to develop novel serine hydrolases.
These are enzymes that catalyze the breaking of ester bonds using water!
While that sounds simple, breaking an ester bond using an enzyme is a 3 step reaction!
And previous attempts at making this type of ester hydrolase from scratch have ended in failure.
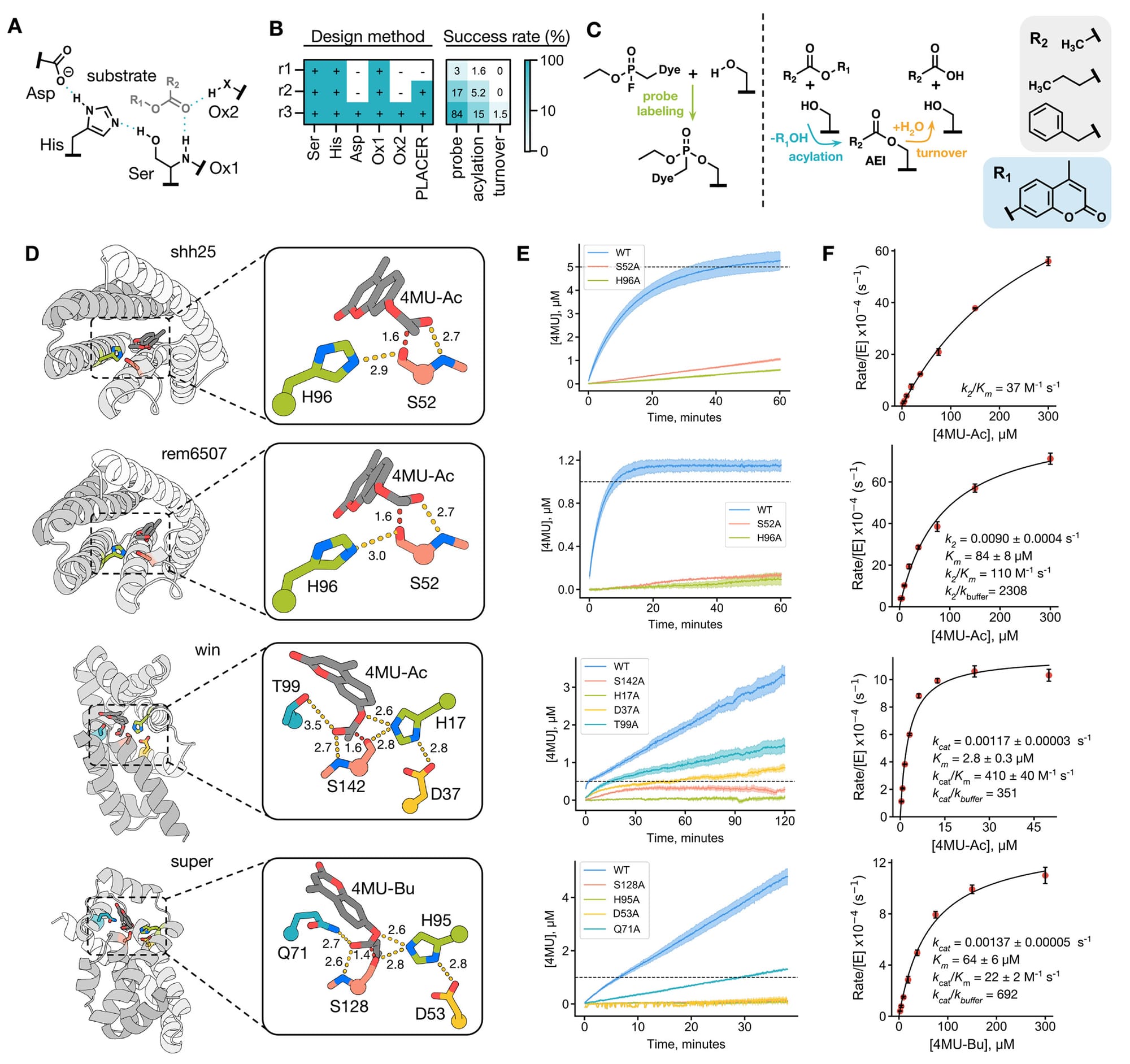
In the figure above, though, you can see that this diffusion based AI ended up finding a few COMPLETELY NEW proteins that actually worked!
A is a model of the active site, B has a table showing what parts of the active site were designed during each round (r1, r2, r3) and then a success table to the right of that showing that only 1.5% of tested enzymes could complete the multi-step (turnover) reaction!, C shows the 3 phases of the reaction and substrates, D shows 4 enzyme structures with a zoom in of their active sites (folded in alphafold 2), E shows the activity of these 4 enzymes - The first two were made in design round 1 and have a plateau which means they didn’t make it past the first step of the reaction while the bottom two enzymes don't show a plateau because they could complete the entire reaction cycle (these are the 2 enzymes that worked in design round 3!), and F is a Michaelis-Menten plot of the catalytic activity of each enzyme.
The researchers went on to show that they could further improve the catalytic activity of the round 3 enzymes and they were able to create at least one that had similar activity as a naturally occurring serine hydrolase.
But the big step forward here is that these newly designed hydrolases have completely different folded structures than their biological counterparts meaning that AI could soon aid in the development of totally novel proteins.
One thing to keep in mind here though is that this AI didn’t just spit out immediately useful structures!
There was a lot of screening that needed to be done here.
Each design round ended with ~200 candidates that were expressed in e. Coli and then tested for their catalytic activity before being used to seed the next design round.
This exquisitely highlights the need for validating the outputs of the solutions that come out of any generative AI model.
But the future of AI assisted protein design looks promising!
